To better understand the science of Bio-hydrox it is best to start from the begining.
Fenton's Reagent
A biologist named H.J.H. Fenton discovered in 1894 that some metals have special oxygen transfer properties which improve the use of Hydrogen Peroxide, and that Iron has a stronger catalytic power to generate highly reactive hydroxyl radicals (.OH). He noted that these oxygen species augment the effects of the regular oxidation process. Since this discovery, the Iron catalyzed hydrogen peroxide has been called Fenton’s reaction. With time, catalyzed oxidation came to be known as advanced oxidation.
Many years later it was identified that not only Iron salt, but other transition metal ions and their complexes in their lower oxidation states could reproduce the reactivity of Fenton reagent. The different combinations of these metal compounds with H2O2 were named “Fenton-like” or “Modified Fenton”.
Bio-hydrox is a modified Fenton’s reagent with a novel chemistry of mineral oxychlorides stabilized in a water based solution.
Advanced Oxidation Processes
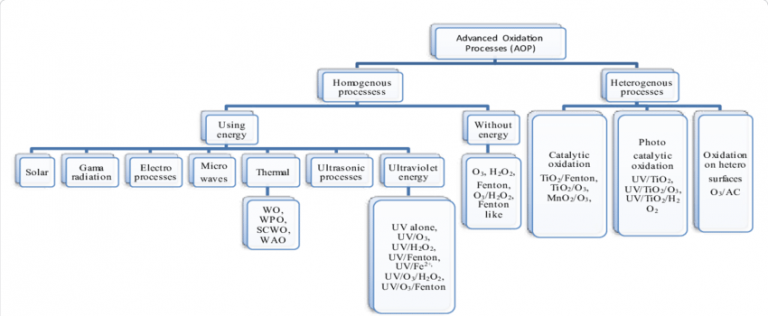
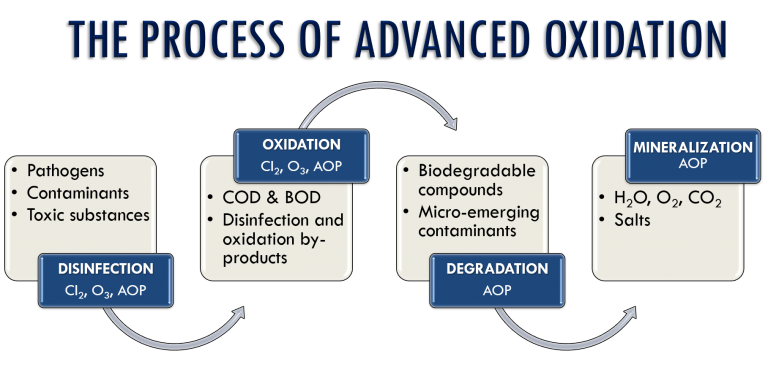
Advanced oxidation processes (AOPs) were first proposed in the 1980s for drinking water treatment and later were widely studied for treatment of different wastewaters. In a general definition, physicochemical procedures which promote in situ generation of free hydroxyl radicals as highly oxidative reagents for the decomposition of pollutants in water or air are described as “advanced oxidation processes”. These oxidation processes begin with one or a combination of any three different reagents: ozone, hydrogen peroxide or an alternate source of oxygen, like Sodium Hypochlorite. They are implemented either combined with each other or applied with UV irradiation and/or various kinds of catalysts homogeneously and heterogeneously. Due to the generation of increased amounts of OH radicals, combination of two or more AOPs usually leads to higher oxidation rates.
During the process of treatment of water, hydroxyl radicals are generated in sufficient quantity to effect a significant change at the sub-molecular level of contaminants, pollutants and recalcitrant and refractory matter, and achieve broad water purification. Depending on the chemical structure of the pollutant molecules, AOPs mineralize them into ultimately harmless substances like CO2 and H2O.
Advanced oxidation processes extend to the oxidative processes with sulfate, permanganese, titanium, and other radicals. For potable water treatment we will refer exclusively to hydroxyl radicals.
- Complex chain of fast reactions that generate enough hydroxyl radicals to produce a significant change at the molecular level.
- In sufficient amounts it can achieve complete mineralization.
- Primarily, non-selective. Simultaneous oxidation of diverse contaminants.
- The subsequent chain of chemical reactions can regenerate oxidizing agents and stay reactive.
- Concentration, not contact time, is the basis of system design.
- Do not introduce any new hazardous substances into the water.
- The increased dissolved oxygen content and lasting residual reactivity improve the quality of the receiving body of water.
- They are evaluated based on results. Mechanism of action is too complex, too fast and not totally understood.
Hydroxyl Radical
OH● is the most reactive oxidizing agent in water treatment. It is very nonselective in its behavior and rapidly reacts with numerous species. The attack by the OH radical, in the presence of oxygen, initiates a complex cascade of oxidative reactions leading to mineralization of the organic compound. Hydroxyl radicals attack organic pollutants through four basic pathways: radical addition, hydrogen abstraction, electron transfer, and radical combination. The exact routes of these reactions are still not quite clear.
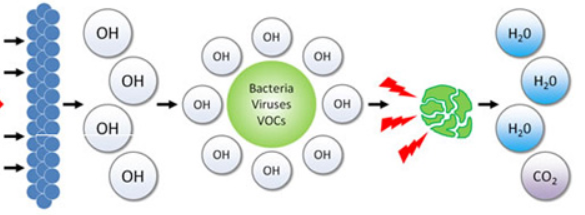
Transition mineral catalyzed reactions dissolved in water offer many advantages. Water is readily available and inexpensive; it is not flammable or explosive. Consequently, the reaction processes are inherently safer and scalable.
Bio-hydrox has the highest yield production of hydroxyl radicals among all AOPs. It also generates large amounts of oxygen ions (reactive oxidant), and superoxide, hydroperoxide anions and perhydroxyl radicals (reactive reductants).
The Reactive Oxygen Species (ROs) released in the process are continually interchanging electrons and regenerating as well as producing new ROs.
The synergy derived from this redox (oxidation-reduction) equilibrium, increases the electrochemical potential higher than any of the reactive species acting alone. This redox activity is perpetuated by the transition minerals catalysts stabilized in solution and thus supports long residual protection.
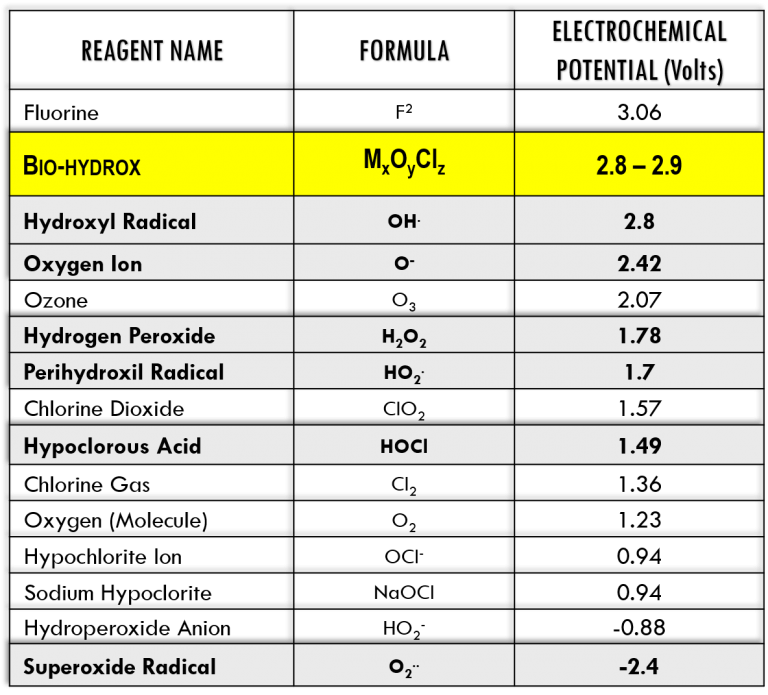
The applications of Bio-hydrox are controlled by oxidation-reduction potential, also called redox potential or ORP. It is an indication of the purity of the water and its capacity to neutralize contaminants based on the potential to oxidize these contaminants. An ORP meter will provide a direct and immediate reading of the quality of the water. The reaction time of an advanced oxidation process is measured in fractions of Nano-Seconds and ORP is the most effective method for monitoring.
In 1971 a 700 mV ORP was adopted by the World Health Organization (WHO) as a standard for drinking water.